[Published] 2023/4/18 [Last updated] 2023/10/20
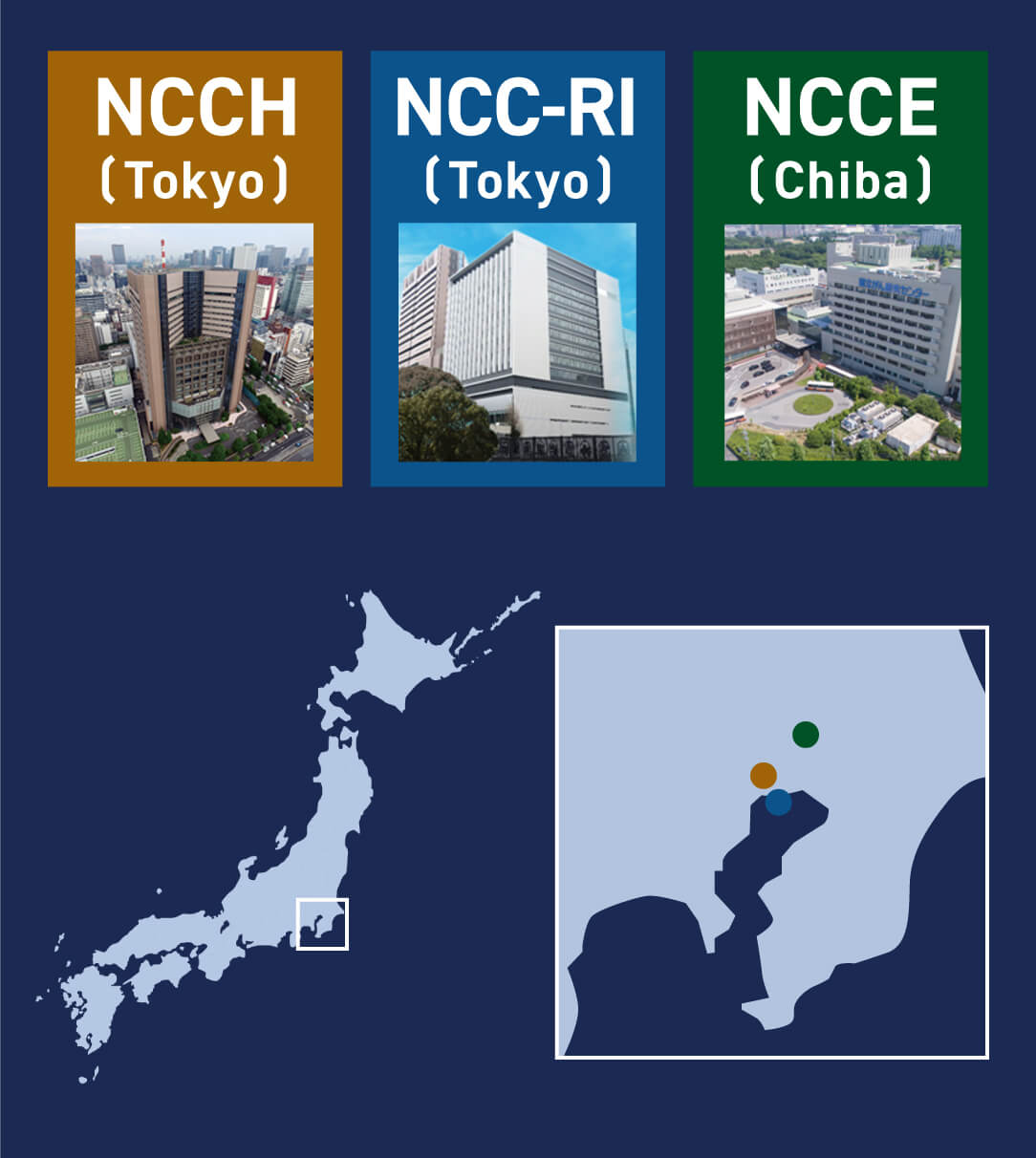
NCC in Japan
- National Cancer Center (NCC) is a leading cancer treatment and research center in Japan.
- NCC has two hospitals and one Research Institute (RI).
- NCCH (Tokyo) and NCCE (Chiba) have 570 and 460 in-patient beds, respectively (total; 1000).
- Each hospital has similar performance according to the in-patient capacity.
- In NCC-RI, fundamental basic research is main expertise. And also, a lot of translational researches are ongoing in collaboration with academia, pharma in Japan as well as overseas.
Our Vision & Strengths
As a One-Stop-Shop Partner for Early R&D:
- Early development of first/best-in-class drugs and novel therapies by participating in novel global FIH phase I trials.
- Integrate nationwide genomics operation from foundational basic science to translational and prevision medicine at phase I clinic.
- Tight link to NCCRI in order to collaborate with external stakeholders/partners involved in early phase drug development and translational research (academic centers, consortia and pharmaceutical companies).
Industry Sponsored trials (ISTs) and Investigator Initiated Trials (IITs) in NCCH
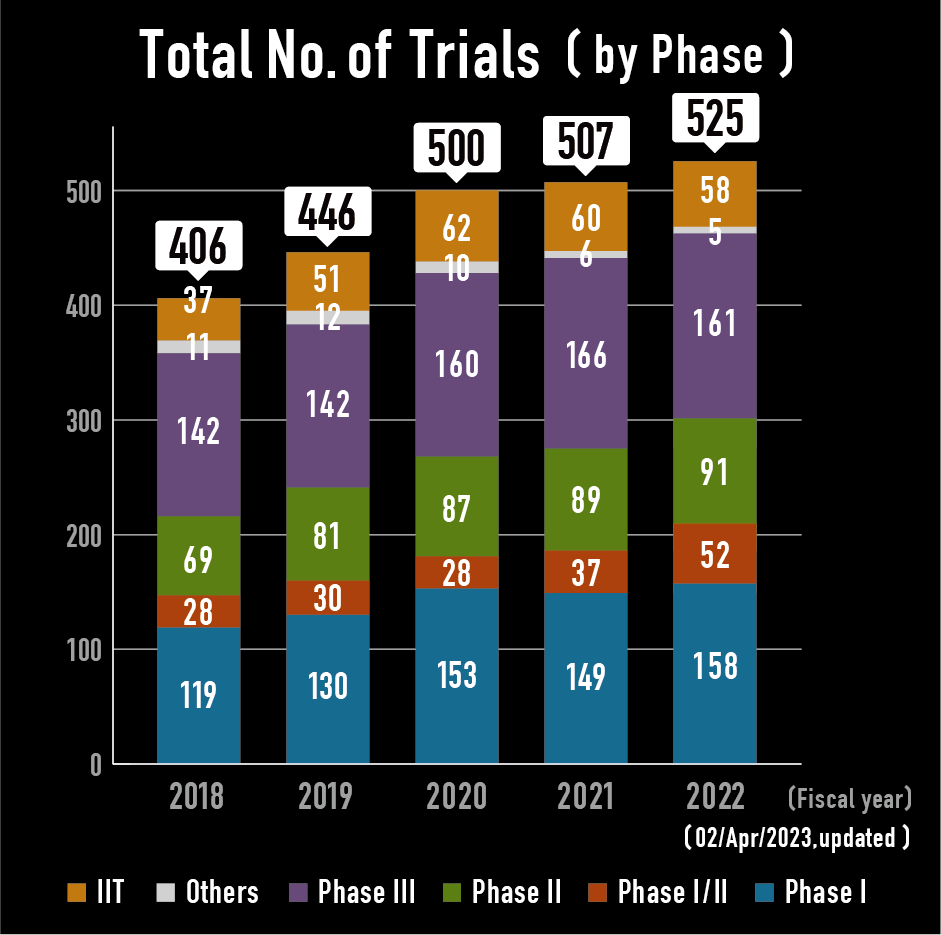
- 90% of trials are industry sponsored trials.
- 60% of trials are global trial.
- High priority for phase I and FIH trials
- IIT is mainly focused on rare cancer and pediatric malignancies.
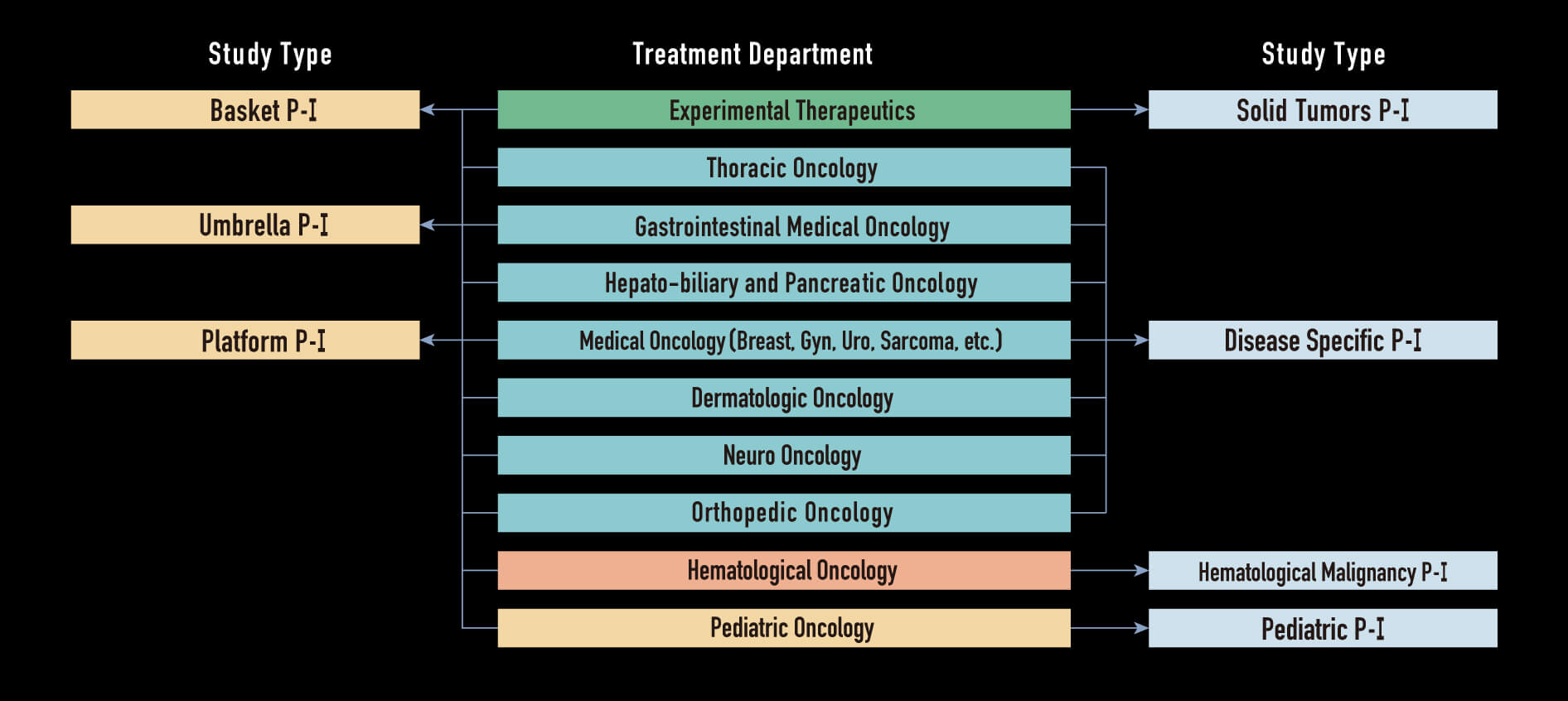
Current Phase I trial, highly diversified
Multiple type phase I trials
- Solid tumors P-I
- Disease specific P-I
- Hematological malignancy P-I
- Pediatric P-I
- Basket P-I
- Umbrella P-I
- Platform P-I
Essential
- NGS screening
- Serial tumor biopsies
Large Scale Trial
- Backfill cohort
- Expansion cohort
Structure for Early Drug Development in NCCH
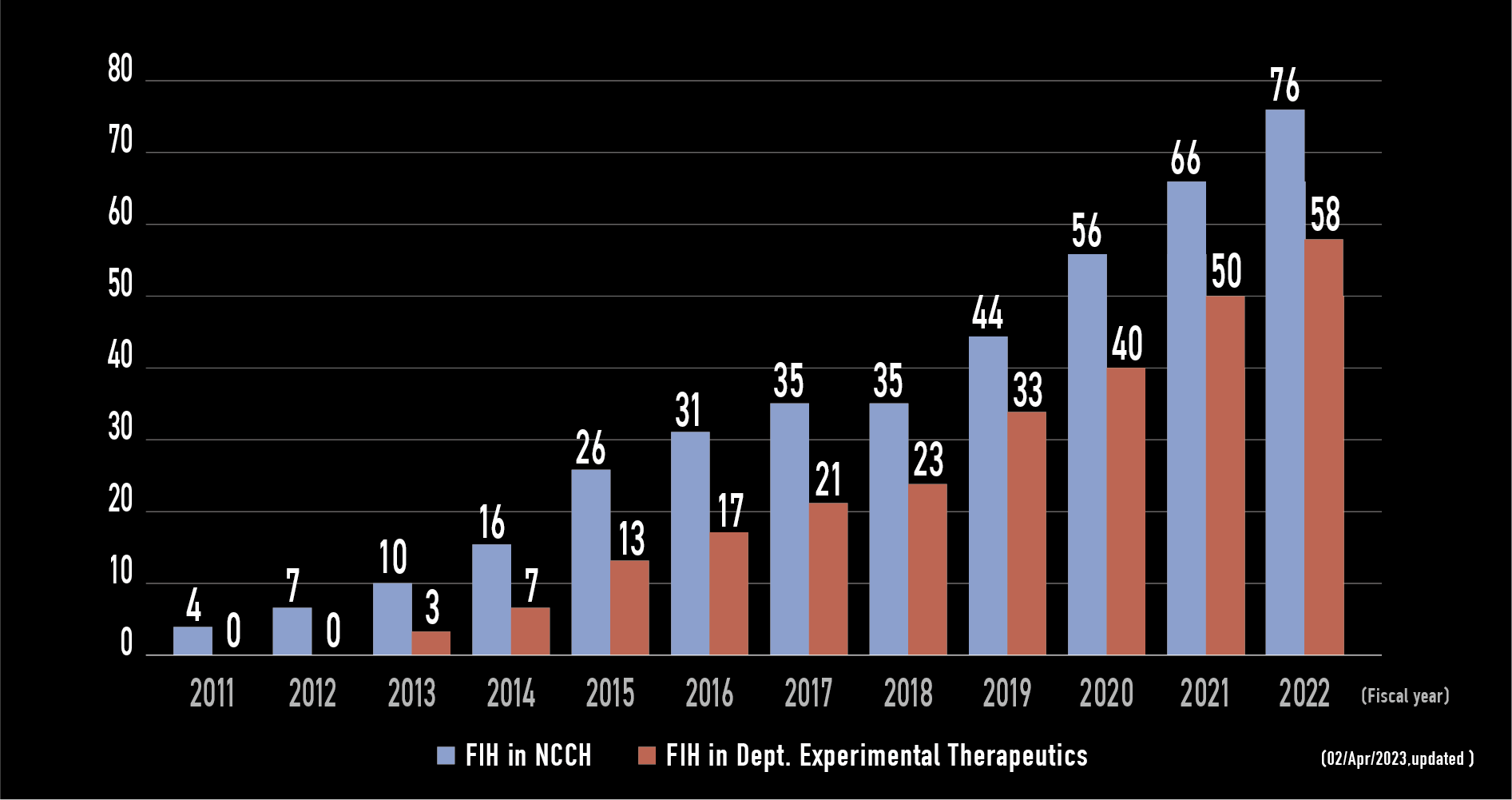
FIH trials in NCCH
- In 2013, early drug development for solid tumors are consolidated to Dept. Experimental Therapeutics.
- The expertise of Dept. Experimental Therapeutics is P-I and FIH for solid tumors regardless of tumor types.
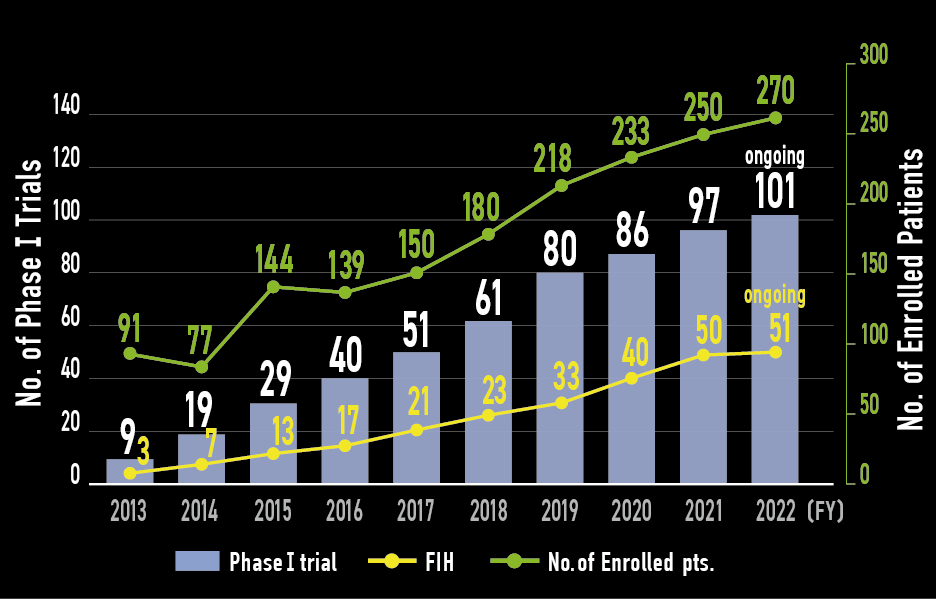
Phase I trials
- In 2019, NGS has been covered by health insurance.
- 95% of patients, NGS profiling has been done before P-I entry.
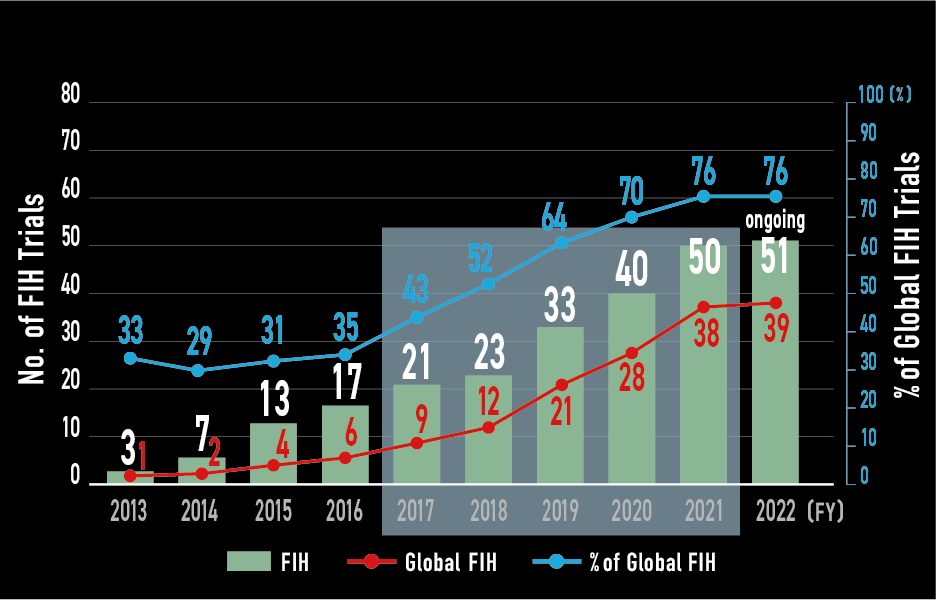
FIH trials
- In 2021, 8 (21%) out of 38 FIH achieved global FSI.
- 26 (68%) out of 38 FIH joined from Initial cohort.
Key to Achieve Global FSI and Initial Cohort Commitment
- In NCCH
- ● Recruit performance
● High frequency (95%) of NGS screening prior to visit phase I clinic
● Rapid preparation from IRB submission to contract
・Ave. 29.0 days in FY2021
- Outstanding performance
in Japan-team - Abbvie, Novartis, Boehringer, Eli Lilly, etc.
Our Advantage
- Close relationship with regulatory agency (PMDA)
- ● More than 10 investigators have experienced human exchange program.
- TR available
- ● Research institute is within the same campus.
● A lot of human bio-samples available for collaborative research
- NGS screening covered by health insurance
- ● No need to prescreening for each trial enrollment
- Center for Cancer Genomics and Advanced Therapeutics (C-CAT)
- ● National genomic database is available for pharma, biotech and academia.
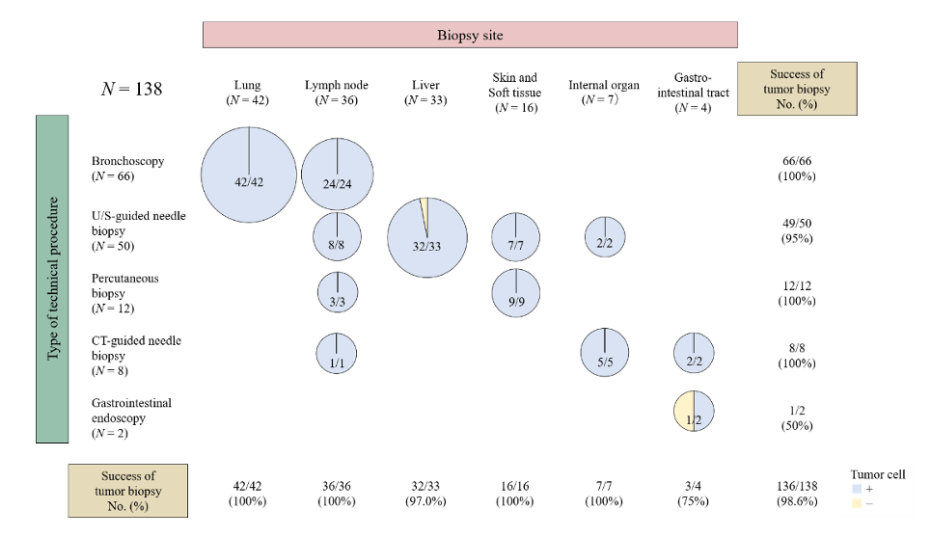
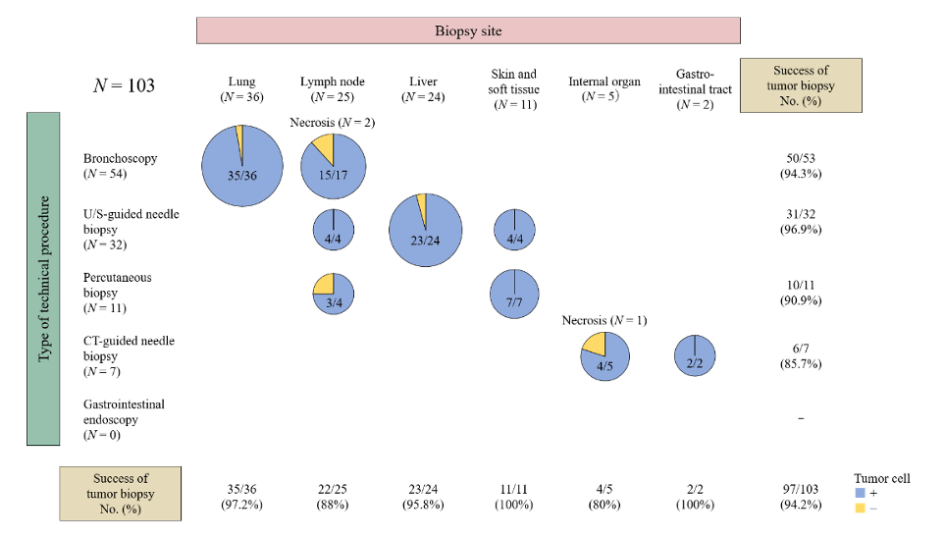
Serial Tumor Biopsy in FIH

- Success rates of
- Pre-treatment biopsy: 98.6%
- On-treatment biopsy: 94.2%
Pre-treatment Biopsy
On-treatment Biopsy
Another Commitment in Phase I and FIH
New additional development proposal