[Published] 2019/8/28 [Last updated] 2023/8/21
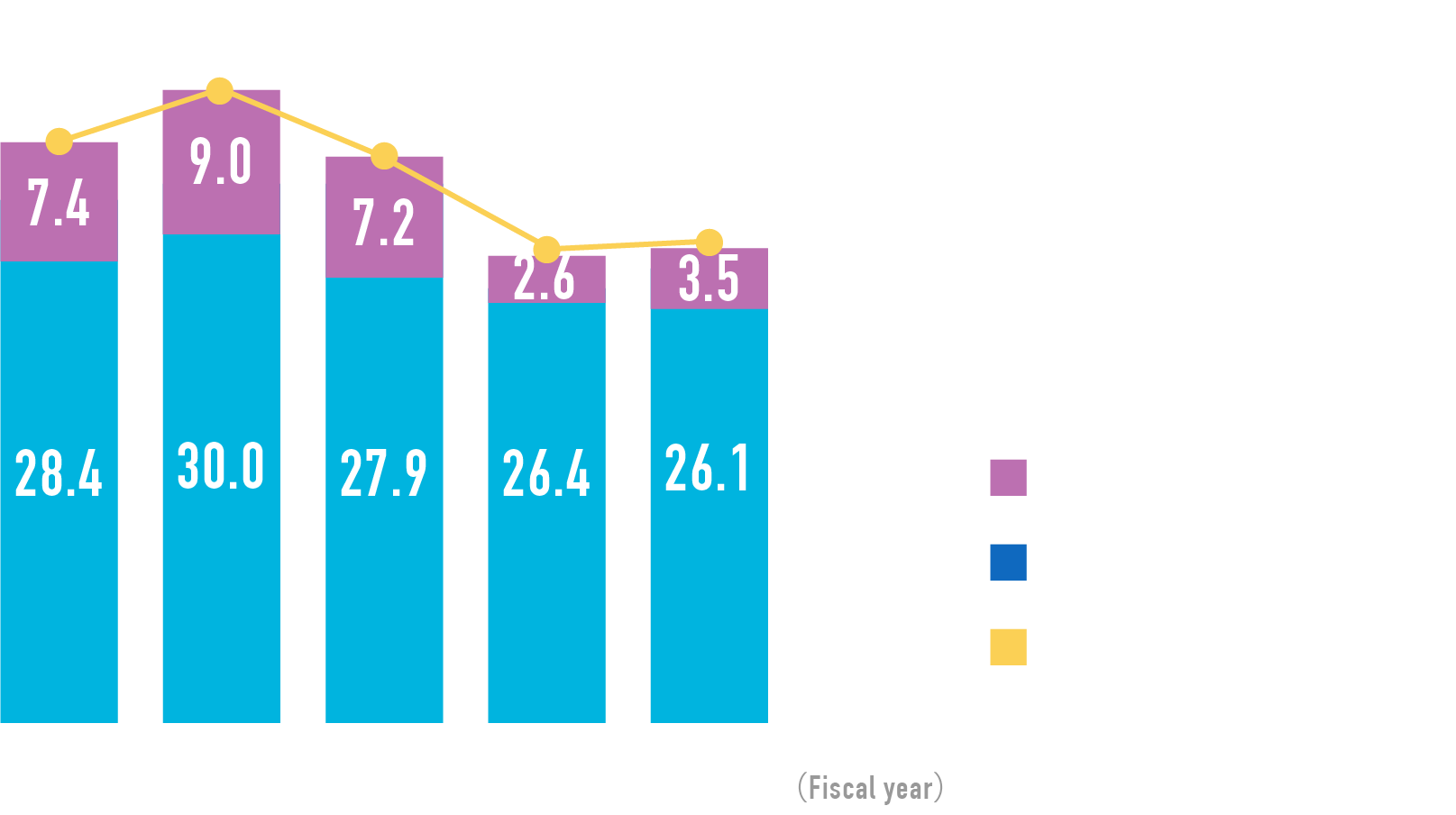
Recent year in review of our phase 1 program, the total number of patients enrolled into trials has been exceeded more than 270 and the total number of both ongoing phase 1 trials and the newly opened trials are increasing as figure is indicating.
- 101
- Number of ongoing
Phase 1 trials
- 270人
- Number of Patients per year
enrolled in phase 1 trials
- 39
- Number of ongoing FIH
phase 1 trials
Recent turnover lead time from the institutional IRB application to the study contract at National Cancer Center Hospital has been remarkably shortened which enables the sponsor to get new clinical trials open more fast.
Talking about the enhanced capabilities through collaboration, a fair number of global pharmaceutical and National Cancer Center Hospital expanded their comprehensive alliance partnership to bring innovative treatment to cancer patients by developing novel oncology therapies.
With an experience of more than 10 years in conducting the phase 1 trials including the First-in-Human phase 1 trials, our phase 1 program has been recognized as a key top recruiting center of the numerous number of the global phase 1 trials as well as the Center of Excellence for new drug development in Asia.
- AbbVie
- Abnova
- AstraZeneca
- Bayer
- Boehringer Ingelheim
- Bristol-Myers Squibb
- Canon Medical Systems
- Daiichi Sankyo
- FUJITSU
- GSK
- Kyowa Hakko Kirin
- Merck
- Merck BioPharma
- Olympus
- ONO Pharmaceutical
- Pfizer
- Sanofi
- SHIMADZU
- Sysmex
- Takeda Oncology
- Thermo Fisher Scientific






